REVIEW PAPER
The safety of using oral nicotine pouche – consideration of their effects on general health, oral mucosa and periodontal diseases, comparing them to snus and other nicotinecontaining products
1
Department of Oral Mucosal and Periodontal Diseases, Medical University, Łódź, Poland
These authors had equal contribution to this work
Corresponding author
Amelia Weronika Rusiecka
Department of Oral Mucosal and Periodontal Diseases Medical University of Łódź, Pomorska 251, 92-217, Łódź, Poland
Department of Oral Mucosal and Periodontal Diseases Medical University of Łódź, Pomorska 251, 92-217, Łódź, Poland
J Pre Clin Clin Res. 2024;18(3):224-230
KEYWORDS
addictioninflammationriskbacteriateethtoxicityperiodontiumsnusoral nicotine pouchesnicotine pouches and mucosa
TOPICS
ABSTRACT
Introduction and objective:
Oral nicotine pouches (ONPs) are tobacco-free and non-combustible products that are pouch-shaped and fit between the alveolar process and the upper lip. Through their use, nicotine is absorbed into the body through the oral mucosa. Their harmfulness is significantly less than that of traditional cigarettes because they do not require combustion.
Review methods:
A review was carried out of the literature in English from 2010–2023 using the PubMed database.
Brief description of the state of knowledge:
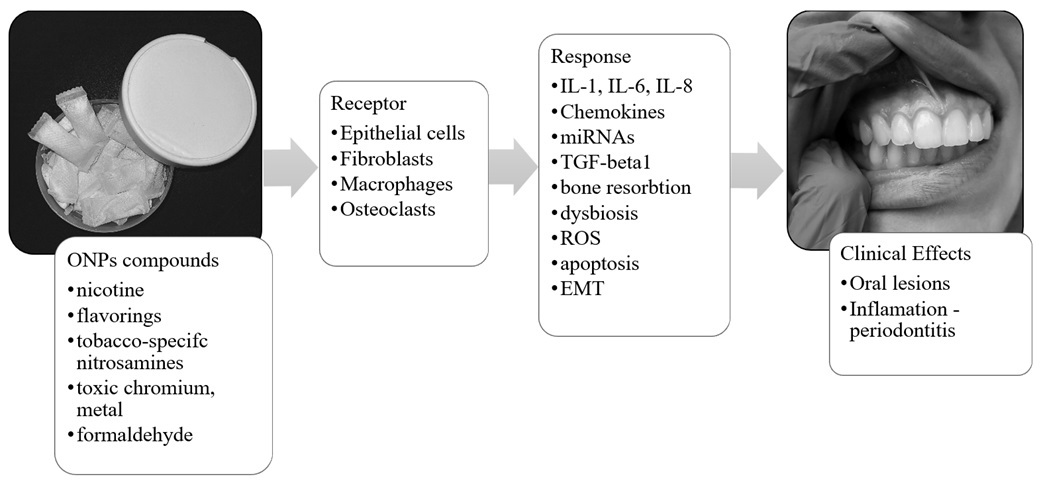
Research indicates ONPs have lower cytotoxicity compared to snus and cigarettes, with some studies suggesting reduced harm-related biomarkers. However, they still pose health risks. The unregulated status and accessibility of nicotine pouches, especially to youth, underscore the need for awareness of their potential dangers. It is important to emphasize their potential negative effects on the oral mucosa and periodontium, as well as on the body, due to their content of nicotine and other potentially dangerous substances. ONPs are suspected of contributing to mucosal lesions, gingival recession, alveolar bone loss, and increased mediators of periodontitis like MMP- 1, MMP-3, and IL-1. Application of ONP to human gingival epithelial cells (HGEPp) resulted in elevated levels of lactate dehydrogenase (LDH), ROS, and inflammatory cytokines (TNF-α, IL-6, and IL-8). ONPs contain low levels of tobacco-derived carcinogens, and may include substances classified by the IARC as potentially carcinogenic.
Summary:
Due to the short time the product has been on the market and narrow period of widespread use, the effects of ONP on human health cannot be predicted with certainty. It is very important to conduct further research in several areas regarding the health effects of ONP and potential consequences that may occur in the future.
Oral nicotine pouches (ONPs) are tobacco-free and non-combustible products that are pouch-shaped and fit between the alveolar process and the upper lip. Through their use, nicotine is absorbed into the body through the oral mucosa. Their harmfulness is significantly less than that of traditional cigarettes because they do not require combustion.
Review methods:
A review was carried out of the literature in English from 2010–2023 using the PubMed database.
Brief description of the state of knowledge:
Research indicates ONPs have lower cytotoxicity compared to snus and cigarettes, with some studies suggesting reduced harm-related biomarkers. However, they still pose health risks. The unregulated status and accessibility of nicotine pouches, especially to youth, underscore the need for awareness of their potential dangers. It is important to emphasize their potential negative effects on the oral mucosa and periodontium, as well as on the body, due to their content of nicotine and other potentially dangerous substances. ONPs are suspected of contributing to mucosal lesions, gingival recession, alveolar bone loss, and increased mediators of periodontitis like MMP- 1, MMP-3, and IL-1. Application of ONP to human gingival epithelial cells (HGEPp) resulted in elevated levels of lactate dehydrogenase (LDH), ROS, and inflammatory cytokines (TNF-α, IL-6, and IL-8). ONPs contain low levels of tobacco-derived carcinogens, and may include substances classified by the IARC as potentially carcinogenic.
Summary:
Due to the short time the product has been on the market and narrow period of widespread use, the effects of ONP on human health cannot be predicted with certainty. It is very important to conduct further research in several areas regarding the health effects of ONP and potential consequences that may occur in the future.
Rusiecka AW, Szymacha K, Lewkowicz N. The safety of using oral nicotine pouches – consideration of their effects on the general health, oral mucosa and periodontal diseases, comparing them to snus and other nicotine-containing products. J Pre-Clin Clin Res. 2024; 18(3): 224–230. doi: 10.26444/jpccr/189645
REFERENCES (37)
1.
Harlow AF, Vogel EA, Tackett AP, et al. Adolescent Use of Flavored Non-Tobacco Oral Nicotine Products. Pediatrics. 2022 Sep 1;150(3):e2022056586. https://doi.org/10.1542/peds.2....
2.
Fagerström K. Can alternative nicotine products put the final nail in the smoking coffin? Harm Reduct J. 2022 Dec 1;4–9 19(1):131. https://doi.org/10.1186/s12954....
3.
Chapman F, McDermott S, Rudd K, et al. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic, pharmacodynamic and safety and tolerability profiles of tobacco-free oral nicotine pouches relative to cigarettes. Psychopharmacology 2022 Sep;239(9):2931–2943. https://doi.org/10.1007/s00213....
4.
Bishop E, East N, Bozhilova S, et al. An approach for the extract generation and toxicological assessment of tobacco-free ‘modern’ oral nicotine pouches. Food Chem Toxicol. 2020 Nov;145:111713. https://doi.org/10.1016/j.fct.....
5.
Azzopardi D, Liu C, Murphy J. Chemical characterization of tobacco-free “modern” oral nicotine pouches and their position on the toxicant and risk continuums. Drug Chem Toxicol. 2022 Sep;45(5):2246–2254. https://doi.org/10.1080/014805....
6.
East N, Bishop E, Breheny D, et al. A screening approach for the evaluation of tobacco-free ‘modern oral’ nicotine products using Real Time Cell Analysis. Toxicol Rep. 2021 Feb 25;8:481–488. https://doi.org/10.1016/j.toxr....
7.
Clarke E, Thompson K, Weaver S, et al. Snus: a compelling harm reduction alternative to cigarettes. Harm Reduct J. 2019 Nov 27: 1–17;16(1):62. https://doi.org/10.1186/s12954....
8.
Ramboll. Systematic review and update of the literature on the health effects of Swedish snus. https://www.swedishmatch.com/g... (access: 2020.10.30).
9.
Back S, Masser AE, Rutqvist LE, et al. Harmful and potentially harmful constituents (HPHCs) in two novel nicotine pouch products in comparison with regular smokeless tobacco products and pharmaceutical nicotine replacement therapy products (NRTs). BMC Chem. 2023 Mar 3: 1–10;17(1):9. https://doi.org/10.1186/s13065....
10.
Azzopardi D, Haswell LE, Frosina J, et al. Assessment of biomarkers of exposure and potential harm, and physiological and subjective health measures in exclusive users of nicotine pouches and current, former and never smokers. Biomarkers. 2023 Feb;28(1):118–129. https://doi.org/10.1080/135475....
11.
Mantey DS, Omega-Njemnobi O, Montgomery L. Flavored tobacco use is associated with dual and poly tobacco use among adolescents. Addict Behav. 2019 Jun;93:269–273. https://doi.org/10.1016/j.addb....
12.
Lund KE, Vedøy TF. A conceptual framework for assessing the public health effects from snus and novel non-combustible nicotine products. Nordisk Alkohol Nark. 2021 Dec;38(6):586–604. https://doi.org/10.1177/145507....
13.
Leventhal AM, Cho J, Vogel EA, et al. Differences in intention to use flavored oral nicotine products among young adult e-cigarette users and non-users. Prev Med Rep. 2022 Oct 17;30:102027. https://doi.org/10.1016/j.pmed....
14.
Delnevo CD, Hrywna M, Miller Lo EJ, et al. Examining Market Trends in Smokeless Tobacco Sales in the United States: 2011–2019. Nicotine Tob Res. 2021 Aug 4;23(8):1420–1424. https://doi.org/10.1093/ntr/nt....
15.
Mallock-Ohnesorg N, Rinaldi S, Malke S, et al. Oral nicotine pouches with an aftertaste? Part 1: screening and initial toxicological assessment of flavorings and other ingredients. Arch Toxicol. 2023 Sep;97(9):2357–2369. https://doi.org/10.1007/s00204....
16.
Kyriakides S, Parliamentary question – E-002498/2023(ASW). https://www.europarl.europa.eu... (access: 2023.10.25).
17.
Szulc M, Coraz bliżej regulacji dla saszetek nikotynowych. Dziennik Gazeta Prawna. https://podatki.gazetaprawna.p.... (access: 2023.11.25).
18.
Rensch J, Edmiston J, Wang J, et al. Randomized, Controlled Study to Assess Changes in Biomarkers of Exposures Among Adults Who Smoke That Switch to Oral Nicotine Pouch Products Relative to Continuing Smoking or Stopping All Tobacco Use. J Clin Pharmacol. 2023 Oct;63(10):1108–1118. https://doi.org/10.1002/jcph.2....
19.
McEwan M, Azzopardi D, Gale N, et al. Randomised Study to Investigate the Nicotine Pharmacokinetics of Oral Nicotine Pouches and a Combustible Cigarette. Eur J Drug Metab Pharmacokinet. 2022 Mar;47(2):211–221. https://doi.org/10.1007/s13318....
20.
Miluna S, Melderis R, Briuka L, et al. The Correlation of Swedish Snus, Nicotine Pouches and Other Tobacco Products with Oral Mucosal Health and Salivary Biomarkers. Dent J (Basel). 2022 Aug 17;10(8):154. https://doi.org/10.3390/dj1008....
21.
Kopperud SE, Ansteinsson V, Mdala I, et al. Oral lesions associated with daily use of snus, a moist smokeless tobacco product. A cross-sectional study among Norwegian adolescents. Acta Odontol Scand. 2023 Aug;81(6):473–478. https://doi.org/10.1080/000163....
22.
Kharazmi M, Carlsson AP, Hallberg P, et al. Surgical approach to snus-induced injury of the oral mucosa. J Oral Sci. 2014 Mar;56(1):91–4. https://doi.org/10.2334/josnus....
23.
Ye D, Rahman I. Emerging Oral Nicotine Products and Periodontal Diseases. Int J Dent. 2023 Feb 10;2023:9437475. https://doi.org/10.1155/2023/9....
24.
Faizi N, Kazmi S. Universal health coverage – There is more to it than meets the eye. J Family Med Prim Care. 2017 Jan-Mar;6(1):169–170. https://doi.org/10.4103/jfmpc.....
25.
Underner M, Perriot J, Peiffer G. Le snus [Smokeless tobacco]. Presse Med. 2012 Jan;41(1):3–9. French. https://doi.org/10.1016/j.lpm.....
26.
Mishra A, Chaturvedi P, Datta S, et al. Harmful effects of nicotine. Indian J Med Paediatr Oncol. 2015 Jan-Mar;36(1):24–31. https://doi.org/10.4103/0971-5....
27.
Leite FRM, Nascimento GG, Scheutz F, et al. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am J Prev Med. 2018 Jun;54(6):831–841. https://doi.org/10.1016/j.amep....
28.
Wang XJ, Liu YF, Wang QY, et al. Functional expression of alpha 7 nicotinic acetylcholine receptors in human periodontal ligament fibroblasts and rat periodontal tissues. Cell Tissue Res. 2010 May;340(2):347–55. https://doi.org/10.1007/s00441....
29.
Kim SY, Kang KL, Lee JC, et al. Nicotinic acetylcholine receptor α7 and β4 subunits contribute nicotine-induced apoptosis in periodontal ligament stem cells. Mol Cells. 2012 Apr;33(4):343–50. https://doi.org/10.1007/s10059....
30.
Kang SW, Park HJ, Ban JY, et al. Effects of nicotine on apoptosis in human gingival fibroblasts. Arch Oral Biol. 2011 Oct;56(10):1091–7. https://doi.org/10.1016/j.arch....
31.
Silva H. Tobacco Use and Periodontal Disease-The Role of Microvascular Dysfunction. Biology (Basel). 2021 May 17; 1–27; 10(5):441. https://doi.org/10.3390/biolog....
32.
Du A, Cheng Y, Zhao S, et al. MicroRNA expression profiling of nicotine-treated human periodontal ligament cells. J Oral Sci. 2019 Jun 18;61(2):206–212. https://doi.org/10.2334/josnus....
33.
Shaikh SB, Newton C, Tung WC, et al. Classification, Perception, and Toxicity of Emerging Flavored Oral Nicotine Pouches. Int J Environ Res Public Health. 2023 Mar 3: 1–14 20(5):4526. https://doi.org/10.3390/ijerph....
34.
Mallock N, Schulz T, Luch A,. Levels of nicotine and tobacco-specific nitrosamines in oral nicotine pouches. Tobacco Control 2022. 1–7. https://doi.org/10.1136/tc-202....
35.
Shaikh SB, Tung WC, Pang C, et al. Flavor Classification/Categorization and Differential Toxicity of Oral Nicotine Pouches (ONPs) in Oral Gingival Epithelial Cells and Bronchial Epithelial Cells. Toxics. 2022 Oct 31: 10(11):660. https://doi.org/10.3390/toxics....
36.
Rinaldi S, Pieper E, Schulz T, et al. Oral nicotine pouches with an aftertaste? Part 2: in vitro toxicity in human gingival fibroblasts. Arch Toxicol. 2023 Sep;97(9):2343–2356. https://doi.org/10.1007/s00204....
37.
Jin J, Guo L, VonTungeln L, et al. Smokeless tobacco impacts oral microbiota in a Syrian Golden hamster cheek pouch carcinogenesis model. Anaerobe. 2018 Aug;52:29–42. https://doi.org/10.1016/j.anae....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.