CASE REPORT
Patient diagnosed with mantle cell lymphoma with tumour lysis syndrome – Case Report and literature review
1
Student Research Group / Second Department of Anaesthesiology and Intensive Therapy, Medical University, Lublin, Poland
2
Second Department of Anaesthesiology and Intensive Therapy, Medical University, Lublin, Poland
Corresponding author
Julia Siek
Student Research Group of The Second Department of Anaesthesiology and Intensive Therapy, Medical University, Staszica 16, 20-081 Lublin, Poland
Student Research Group of The Second Department of Anaesthesiology and Intensive Therapy, Medical University, Staszica 16, 20-081 Lublin, Poland
J Pre Clin Clin Res. 2023;17(2):113-116
KEYWORDS
TOPICS
ABSTRACT
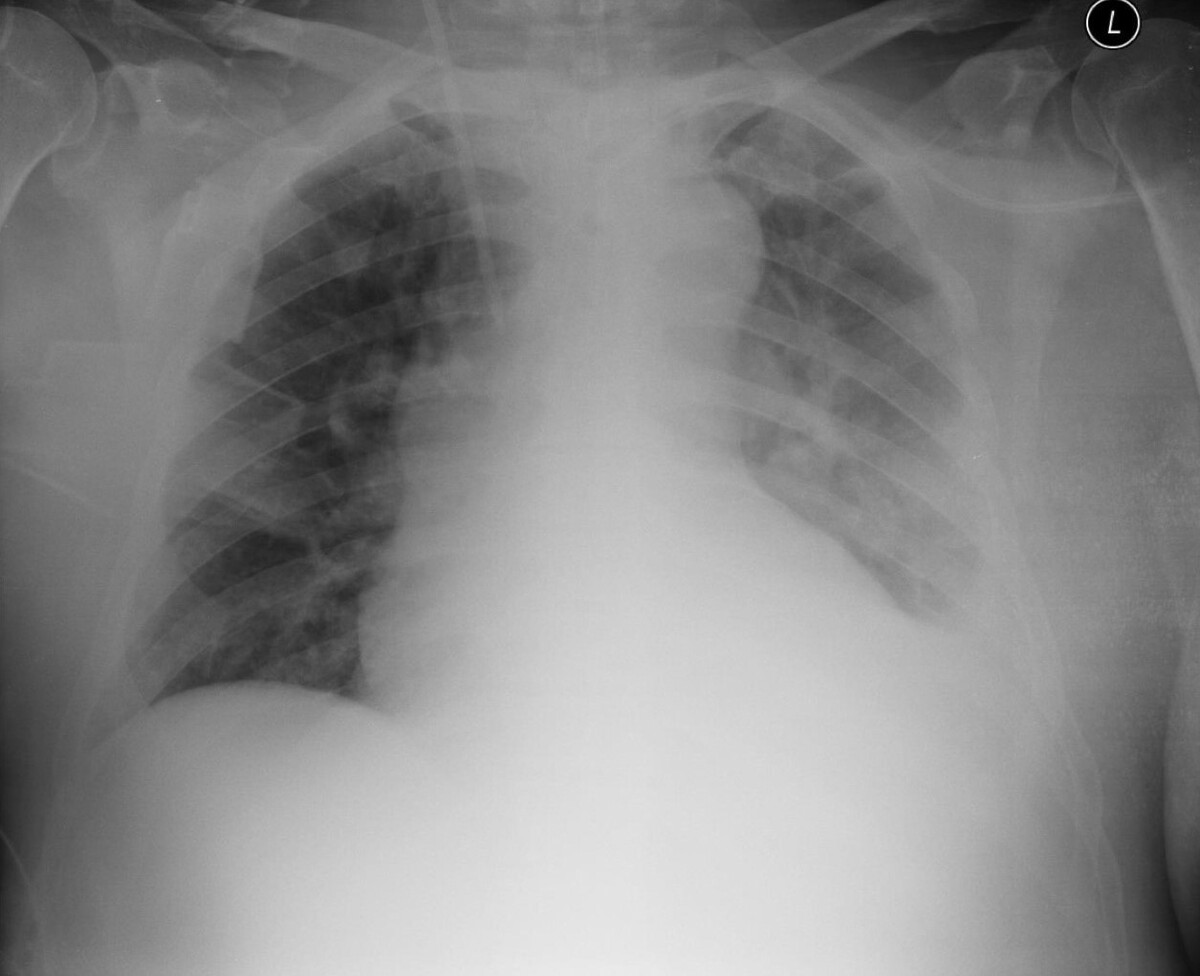
Mantle cell lymphoma (MCL) is an aggressive, rare form of non-Hodgkin lymphoma (NHL). This lymphoma is characterized by the expansion of mature B cells which spread in the bone marrow, blood, lymphatic tissues and extranodal sites. Due to limited treatment options, it is one of the most complex neoplastic diseases of the lymphoid system. The typical age of onset is 60–70 years. Occasionally, tumour lysis syndrome (TLS) can occur due to the cancer cells breaking-down too quickly. Thesudden, intense breakdown of the cancer cells releases large amounts of potassium, purines and phosphates. In the clinical case described by the authors, the patient was diagnosed with MCL in the generalized CS III stage. The patient additionally developed TLS. Previously, the patient had been treated with rituximab at the Department of Haematooncology, and due to respiratory and renal failure, was subsequently admitted to the Intensive Care Unit (ICU) of the University Hospital in Lublin.
Siek J, Sysiak-Sławecka J. Patient diagnosed with mantle cell lymphoma with tumour lysis syndrome- case report and literature review. J Pre-Clin Clin Res. 2023; 17(2): 113–116. doi: 10.26444/jpccr/166084
REFERENCES (19)
1.
Hansen SV, Nyvold CG, Hansen MH. Mantle cell lymphoma and the evidenceof an immature lymphoid component. Leuk Res. 2022;115:106824. doi: 10.1016/j.leukres.2022.106824.
2.
Walewski J. Chłoniak z komórek płaszcza u pacjentów niekwalifikujących się do chemioterapii w wysokich dawkach. Acta Haematol Pol. 2013;104–109.
3.
Kumar A, Eyre TA, Lewis KL, et al. New Directions for Mantle Cell Lymphoma in 2022. Am Soc Clin Oncol Educ Book. 2022;42:1–15. doi:10.1200/EDBK_349509.
4.
Bond DA, Martin P, Maddocks KJ. Relapsed Mantle Cell Lymphoma: Current Management, Recent Progress, and Future Directions. J Clin Med. 2021;10(6):1207. doi:10.3390/jcm10061207.
5.
Kumar A, Sha F, Toure A, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: Progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9(6):50. doi:10.1038/s41408-019-0209-5.
6.
Witzig TE, Inwards D. Acalabrutinib for mantle cell lymphoma. Blood. 2019;133(24):2570–2574. doi:10.1182/blood.2019852368.
7.
Williams SM, Killeen AA. Tumour Lysis Syndrome. Arch Pathol Lab Med. 2019;143(3):386–393. doi:10.5858/arpa.2017-0278-RS8.
8.
Hanel W, Epperla N. Emerging therapies in mantle cell lymohoma. J Hematol Oncol. 2020;13(1):79. doi:10.1186/s13045-020-00914-1.
9.
Epperla N, Hamadani M, Fenske TS, et al. Incidence and survival trends in mantle cell lymphoma. Br J Haematol. 2018;181(5):703–706. doi: 10.1111/bjh.14699.
10.
Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood.2017;130(17):1903–1910. doi:10.1182/blood-2017-04-779736.
11.
Radhakrishnan VS, Lokireddy P, Parihar M, et al. Mantle cell lymphoma: A clinical review of the changing treatment paradigms with the advent of novel therapies, and an insight into Indian data. Cancer Rep (Hoboken). 2022;5(7):e1590. doi:10.1002/cnr2.1590.
12.
Kumar A. What is the role of up-front autologous stem cell transplantation in mantle cell lymphoma? Hematology Am Soc Hematol Educ Program. 2022;(1):155–162. doi:10.1182/hematology.2022000333.
13.
Adeyinka A, Bashir K. Tumour Lysis Syndrome. Treasure Island (FL): StatPearls Publishing; 2022.
14.
Cohen PR, Prieto VG, Kurzrock R. Tumour Lysis Syndrome: Introduction of a Cutaneous Variant and a New Classification System. Cureus. 2021;13(3):e13816. doi:10.7759/cureus.13816.
15.
Puri I, Sharma D, Gunturu KS, et al. Diagnosis and management of tumour lysis syndrome. J Community Hosp Intern Med Perspect. 2020;10(3):269–272. doi:10.1080/20009666.2020.1761185.
16.
Luminais SN, Chen XT, Roman D, et al. Tumour lysis syndrome following ifosfamide monotherapy in metastatic osteosarcoma: a case report and review of the literature. J Med Case Rep. 2022;16(1):252. doi:10.1186/s13256-022-03469-6.
17.
Piwowarczyk P, Kutnik P, Potręć-Studzińska B, et al. Hemoadsorption in isolated conjugated hyperbilirubinemia after extracorporeal membrane oxygenation support. Cholestasis of sepsis: A case report and review of the literature on differential causes of jaundice in ICU patient. Int J Artif Organs. 2019;42(5):263–268. doi:10.1177/0391398819834012.
18.
Kutnik P, Szczukocka M, Borys M, Czuczwar M. Procalcitonin dynamics, lactates, and haemoglobin serum levels might be a useful predictive tool of mortality in patients undergoing veno-venous extracorporeal oxygenation membrane support. Single centre experience. Anaesthesiol Intensive Ther. 2019;51(5):343–347. doi:10.5114/ait.2019.90235.
19.
Stephanos K, Picard L. Pediatric Oncologic Emergencies. Emerg Med Clin North Am. 2018;36(3):527–535. doi:10.1016/j.emc.2018.04.007.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.