RESEARCH PAPER
Isobolographic in vitro interactions of fluconazole with citrus essential oils against Cladosporium cladosporioides
1
Medical University, Lublin, Poland
J Pre Clin Clin Res. 2021;15(1):15-19
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Cladosporium is one of the most abundant genera of environmental fungi worldwide and a very common respiratory allergen. To-date, many C. cladosporoides infections have been identified. Risk factors for C. cladosporioides infection are primarily injuries, metabolic disorders, organ transplantation and autoimmune diseases, among others.
Objective:
The aim of the study was to assess the type of pharmacodynamic interactions between fluconazole and some selected essential oils: orange, mandarin, lemon and grapefruit, in an in vitro study against C. cladosporioides.
Material and methods:
Experiments were carried out using the plate cultivation method. Fluconazole was tested against C. cladosporioides at concentrations ranging from 0.05–3.3 mg / ml, and the activity of essential oils added to PDA medium at concentrations ranging from 1–30%. The dose-effect curves for the collected results were determined with by the log-probit method. Isobolographic analysis of the results allowed determining the type of interactions between fluconazole and the tested essential oils.
Results:
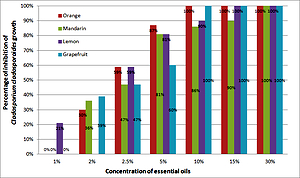
Lemon essential oil was the most active, and in a concentration of 1% it inhibited the growth of C. cladosporioides by 21%. Isobolographic analysis showed that the combination of fluconazole with orange and grapefruit essential oil had an additive interaction, and with mandarin and lemon – an additive interaction with a tendency to synergy in the plate culture test for C. cladosporioides.
Conclusions:
The use of isobolographic analysis can contribute to the introduction of natural substances with the desired activities into the pharmacotherapy of many infections and diseases. The use of natural substances can also help to reduce the number of side-effects caused by conventional and standard therapies
Cladosporium is one of the most abundant genera of environmental fungi worldwide and a very common respiratory allergen. To-date, many C. cladosporoides infections have been identified. Risk factors for C. cladosporioides infection are primarily injuries, metabolic disorders, organ transplantation and autoimmune diseases, among others.
Objective:
The aim of the study was to assess the type of pharmacodynamic interactions between fluconazole and some selected essential oils: orange, mandarin, lemon and grapefruit, in an in vitro study against C. cladosporioides.
Material and methods:
Experiments were carried out using the plate cultivation method. Fluconazole was tested against C. cladosporioides at concentrations ranging from 0.05–3.3 mg / ml, and the activity of essential oils added to PDA medium at concentrations ranging from 1–30%. The dose-effect curves for the collected results were determined with by the log-probit method. Isobolographic analysis of the results allowed determining the type of interactions between fluconazole and the tested essential oils.
Results:
Lemon essential oil was the most active, and in a concentration of 1% it inhibited the growth of C. cladosporioides by 21%. Isobolographic analysis showed that the combination of fluconazole with orange and grapefruit essential oil had an additive interaction, and with mandarin and lemon – an additive interaction with a tendency to synergy in the plate culture test for C. cladosporioides.
Conclusions:
The use of isobolographic analysis can contribute to the introduction of natural substances with the desired activities into the pharmacotherapy of many infections and diseases. The use of natural substances can also help to reduce the number of side-effects caused by conventional and standard therapies
Wróblewska-Łuczka P. Isobolographic in vitro interactions of fluconazole with citrus essential oils against Cladosporium cladosporioides. J Pre-Clin Clin Res. 2021; 15(1): 15–19. doi: 10.26444/jpccr/132014
REFERENCES (34)
1.
Grinn-Gofroń A, Nowosad J, Bosiacka B, et al. Airborne Alternaria and Cladosporium fungal spores in Europe: Forecasting possibilities and relationships with meteorological parameters. Sci Total Environ. 2019; 25(653): 938–946. doi: 10.1016/j.scitotenv.2018.10.419.
2.
Meng J, Barnes CS, Rosenwasser LJ. Identity of the fungal species present in the homes of asthmatic children. Clin Exp Allergy. 2012; 42: 1448–1458. https://doi.org/10.1111/j.1365....
3.
Bousquet PJ, Chinn S, Janson C, et al. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007; 62: 301–309. ht t ps://doi.org /10.1111/j.1398-9995.20 06.01293.x.
4.
Vincent M, Corazza F, Chasseur C, et al. Relationship between mold exposure, specific IgE sensitization, and clinical asthma: A case-control study. Ann Allergy Asthma Immunol. 2018; 121(3): 333–339. doi: 10.1016/j.anai.2018.06.016.
5.
Fukutomi Y, Taniguchi M. Sensitization to fungal allergens: Resolved and unresolved issues. Allergol Int. 2015; 64(4): 321–31. https://doi.org/10.1016/j.alit....
6.
Okada K, Takizawa K, Maebayashi Y, et al. Ubiquinone systems of the genus Cladosporium and morphologically similar taxa. FEMS Immunol Med Microbiol. 1996; 16: 39–43. https://doi.org/10.1016/S0928-....
7.
Walker C, Muniz MF, Rolim JM, et al. Morphological and molecular characterization of Cladosporium cladosporioides species complex causing pecan tree leaf spot. Genet Mol Res. 2016; 15(3). doi: 10.4238/g mr.15038714.
8.
AlMatar M, Makky EA. Cladosporium cladosporioides from the perspectives of medical and biotechnological approaches. 3 Biotech. 2016; 6(1):4. doi: 10.1007/s13205-015-0323-4.
9.
Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2017; 8(3): 352–358. doi: 10.1080/21505594.2016.1247140.
10.
Ziaee A, Zia M, Goli M. Identification of saprophytic and allergenic fungi in indoor and outdoor environments. Environ Monit Assess. 2018; 190(10): 574. doi: 10.1007/s10661-018-6952-4.
11.
AlMatar M, Makky EA. Cladosporium cladosporioides from the perspectives of medical and biotechnological approaches. 3 Biotech. 2016; 6(1): 4. https://doi.org/10.1007/s13205....
12.
Khan AA, Bacha N, Ahmad B, el at. Synthesis of secondary metabolites by Cladosporium resinae (NRL-6437) under different growth media and chemical inducers and their pharmaceutical activity. Pak J Pharm Sci. 2017; 30(5): 1617–1624.
13.
Zhou YB, Chen P, Sun TT, et al. Acne-like subcutaneous phaeohypho-mycosis caused by Cladosporium cladosporioides: A Rare Case Report and Review of Published Literatures. Mycopathologia. 2016; 181(7–8): 567–573. https://doi.org/10.1007/s11046....
14.
Sang H, Zheng XE, Zhou WQ, et al. A case of subcutaneous phaeohyphomycosis caused by Cladosporium cladosporioides and its treatment. Mycoses. 2012; 55: 195–197. https://doi.org/10.1111/j.1439....
15.
Nath R, Barua S, Barman J, et al. Subcutaneous Mycosis Due to Cladosporium cladosporioides and Bipolaris cynodontis from Assam, North-East India and Review of Published Literature. Mycopathologia. 2015; 180(5– 6): 379–87. doi: 10.1007/s11046-015-9926-x.
16.
Velázquez-Jiménez Y, Hernández-Castro R, Romero-Romero L, et al. Feline Phaeohyphomycotic Cerebellitis Caused by Cladosporium cladosporioides-complex: Case Report and Review of Literature. J Comp Pathol. 2019; 170: 78–85. doi: 10.1016/j.jcpa.2019.05.011.
17.
Adaszyńska M, Swarcewicz M, Markowska-Szczupak A. Comparison of chemical composition and antimicrobial activity of Lavender varieties from Poland. Post Fitoter. 2013; 2: 90–96.
18.
Shafaghat A, Salimi F, Amani-Hooshyar V. Phytochemical and antimicrobial activities of Lavandula officinalis leaves and stems against some pathogenic microorganisms. J Med Plant Res. 2012; 6: 455–460. doi: 10.5897/JMPR11.1166.
19.
Aloui H, Khwaldia K, Licciardello F, et al. Efficacy of the combined application of chitosan and Locust Bean Gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int J Food Microbiol. 2014; 170: 21–28. https://doi.org/10.1016/j.ijfo....
20.
Budzyńska A, Więckowska-Szakiel M, Kalemba D, et al. The optimization of methods utilized for testing the antibacterial activity of essential oils. Med Dośw Mikrobiol. 2009; 61: 281–287.
21.
Litchfield JT Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949; 96: 99–113.
22.
Łuszczki JJ. Isobolographic analysis of interaction between drugs with nonparallel dose-response relationship curves: a practical application. Naunyn Schmiedebergs Arch Pharmacol. 2007; 375: 105–114. https://doi.org/10.1007/s00210....
23.
Mousa HA. Prevention and Treatment of Influenza, Influenza-Like Illness, and Common Cold by Herbal, Complementary, and Natural Therapies. J Evid Based Complementary Altern Med. 2017; 22(1): 166–174. doi: 10.1177/2156587216641831.
24.
Tariq S, Wani S, Rasool W, et al. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb Pathog. 2019; 134: 103580. doi: 10.1016/j.micpath.2019.103580.
25.
Ben Hsouna A, Ben Halima N, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017; 16(1): 146. doi: 10.1186/s1294 4- 017- 0487-5.
26.
Smeriglio A, Alloisio S, Raimondo FM, et al. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem Toxicol. 2018; 119: 407–416. doi: 10.1016/j.fct.2017.12.053.
27.
Rombolà L, Amantea D, Russo R, et al. Rational Basis for the Use of Bergamot Essential Oil in Complementary Medicine to Treat Chronic Pain. Mini Rev Med Chem. 2016; 16(9): 721–8. doi: 10.2174/1389557516666160321113913.
28.
Yew SM, Chan CL, Lee KW, et al. A five-year survey of dematiaceous fungi in a tropical hospital reveals potential opportunistic species. PLoS One. 2014; 9(8):e104352. doi: 10.1371/journal.pone.0104352.
29.
de Menezes C, Guerra F, Pinheiro L, et al. Investigation of Melissa officinalis L. Essential Oil for Antifungal Activity against Cladosporium carrionii. International Journal of Tropical Disease and Health. 2015; 8(2): 49–56. https://doi.org/10.9734/IJTDH/....
30.
Zehetner P, Höferl M, Buchbauer G. Essential oil components and cytochrome P450 enzymes: a review. Flavour and Fragrance Journal. 2019; 34(4): 223–240. https://doi.org/10.1002/ffj.34....
31.
Niwa T, Shiraga T, Takagi A. Effect of Antifungal Drugs on Cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 Activities in Human Liver Microsomes. Biol Pharm Bull. 2005; 28(9): 1805–1808. doi: 10.1248/bpb.28.1805.
32.
Ferro BE, Meletiadis J, Wattenberg M, de Jong A, et al. Clofazimine prevents the regrowth of Mycobacterium abscessus and Mycobacterium avium type strains exposed to amikacin and clarithromycin. Antimicrob Agents Chemother. 2015; 60(2): 1097–1105. doi: 10.1128/AAC.02615-15.
33.
Huang L, Dai T, Xuan Y, et al. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: efficacy against bacterial burn infections. Antimicrob Agents Chemother. 2011; 55(7): 3432–3438. doi: 10.1128/AAC.01803-10.
34.
Elefanti A, Mouton JW, Verweij PE, et al. Amphotericin B- and voriconazole-echinocandin combinations against Aspergillus spp.: Effect of serum on inhibitory and fungicidal interactions. Antimicrob Agents Chemother. 2013; 57(10): 4656–4663. doi: 10.1128/AAC.00597-13.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.