CASE REPORT
Epilepsy in a girl with features of CHARGE syndrome – case report and literature review
1
Student Research Group / Department of Paediatric Neurology, Medical University, Lublin, Poland
2
Department of Pediatric Neurology, University Children’s Hospital in Lublin, Medical University, Lublin, Poland
Corresponding author
Natalia Biedroń
The Student Research Group at the Department of Pediatric Neurology, Medical University of Lublin, Poland
The Student Research Group at the Department of Pediatric Neurology, Medical University of Lublin, Poland
J Pre Clin Clin Res. 2025;19(1):15-19
KEYWORDS
TOPICS
ABSTRACT
Introduction:
CHARGE syndrome represents a rare, genetically determined association of birth defects.* The diagnosis of the syndrome is based on the finding of typical clinical symptoms and the detection of mutations in the CHD7 gene.
Case Report:
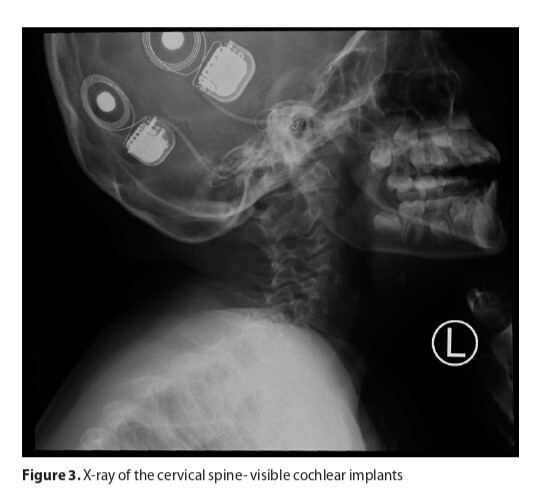
The case of a 13-year-old girl with features of CHARGE syndrome is presented. After birth, the girl was found to have defects that suggested CHARGE syndrome. However, the mutation was not confirmed in the genetic test performed. Despite the presence of all the large CHARGE syndrome criteria in the patient, due to the absence of a genetic mutation, it was not possible to make a complete diagnosis. In addition, the girl had congenital epilepsy, which can occur in CHARGE syndrome but is not characteristic. The onset of epilepsy may also have been independent
Conclusions:
With the discovery of missense de novo variants in WDR37, for which epilepsy and other CHARGE-like symptoms are present, perhaps the final diagnosis of the syndrome in the patient described could have been different
CHARGE syndrome represents a rare, genetically determined association of birth defects.* The diagnosis of the syndrome is based on the finding of typical clinical symptoms and the detection of mutations in the CHD7 gene.
Case Report:
The case of a 13-year-old girl with features of CHARGE syndrome is presented. After birth, the girl was found to have defects that suggested CHARGE syndrome. However, the mutation was not confirmed in the genetic test performed. Despite the presence of all the large CHARGE syndrome criteria in the patient, due to the absence of a genetic mutation, it was not possible to make a complete diagnosis. In addition, the girl had congenital epilepsy, which can occur in CHARGE syndrome but is not characteristic. The onset of epilepsy may also have been independent
Conclusions:
With the discovery of missense de novo variants in WDR37, for which epilepsy and other CHARGE-like symptoms are present, perhaps the final diagnosis of the syndrome in the patient described could have been different
Biedroń N, Kwiatkowski B, Budzeń D, Cichocka A, Ziółkiewicz A, Szukała K, Chrościńska-Krawczyk M. Epilepsy in a girl with features of CHARGE
syndrome – case report and literature review. J Pre-Clin Clin Res. 2025; 19(1): 15–19. doi: 10.26444/jpccr/200539
REFERENCES (31)
1.
Usman N, Sur M. CHARGE Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
3.
Blake KD, Davenport SL, Hall BD, et al. CHARGE association: an update and review for the primary pediatrician. Clin Pediatr. (Phila). 1998;37(3):159–73.
4.
Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet. 2007;15(4):389–99.
5.
Patel DC, Tewari BP, Chaunsali L, et al. Neuron-glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci. 2019;20(5):282–297.
6.
Zuberi SM, Wirrell E, Yozawitz E, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63:1349–1397.
9.
de Geus CM, Free RH, Verbist BM, et al. Guidelines in CHARGE syndrome and the missing link: Cranial imaging. Am J Med Genet C Semin Med Genet. 2017;175(4):450–464.
10.
Traisrisilp K, Chankhunaphas W, Sittiwangkul R, et al. Prenatal Sonographic Features of CHARGE Syndrome. Diagnostics (Basel). 2021;11(3):415.
11.
Millischer AE, Sonigo P, Attie T, et al. Fetal MRI findings in a retrospective cohort of 26 cases of prenatally diagnosed CHARGE syndrome individuals. Prenat Diagn. 2019;39(9):781–791.
12.
Lingam G, Sen AC, Lingam V, et al. Ocular coloboma-a comprehensive review for the clinician. Eye (Lond). 2021;35(8):2086–2109.
13.
Meisner JK, Martin DM. Congenital heart defects in CHARGE: The molecular role of CHD7 and effects on cardiac phenotype and clinical outcomes. Am J Med Genet C Semin Med Genet. 2020;184(1):81–89.
14.
Szleper A, Lachowska M, Wojciechowski T, et al. Detailed analysis of inner ear malformations in CHARGE syndrome patients – correlation with audiological results and proposal for computed tomography scans evaluation methodology. Braz J Otorhinolaryngol. 2024;90(2):101383.
15.
Vesseur AC, Verbist BM, Westerlaan HE, et al. CT findings of the temporal bone in CHARGE syndrome: aspects of importance in cochlear implant surgery. Eur Arch Otorhinolaryngol. 2016;273(12):4225–4240.
16.
Asakura Y, Toyota Y, Muroya K, et al. Endocrine and radiological studies in patients with molecularly confirmed CHARGE syndrome. J Clin Endocrinol Metab. 2008;93(3):920–4.
17.
Consales A, Crippa BL, Colombo L, et al. CHARGE syndrome presenting with persistent hypoglycemia: case report and overview of the main genetic syndromes associated with neonatal hypoglycemia. Ital J Pediatr. 2022;48(1):154.
18.
Wong MT, van Ravenswaaij-Arts CM, Munns CF, et al. Central Adrenal Insufficiency Is Not a Common Feature in CHARGE Syndrome: A Cross-Sectional Study in 2 Cohorts. J Pediatr. 2016;176:150–5.
19.
Specchio N, Wirrell EC, Scheffer IE, et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1398–1442.
20.
Hsu P, Ma A, Wilson M, et al. CHARGE syndrome: a review. J Paediatr Child Health. 2014;50(7):504–11.
21.
Balagura G, Iapadre G, Verrotti A, et al. Moving beyond sodium valproate: choosing the right anti-epileptic drug in children. Expert Opin Pharmacother. 2019;20(12):1449–1456.
22.
Hakami T. Neuropharmacology of Antiseizure Drugs. Neuropsychopharmacol Rep. 2021;41(3):336–351.
23.
Kaplan YC, Demir O. Use of Phenytoin, Phenobarbital Carbamazepine, Levetiracetam Lamotrigine and Valproate in Pregnancy and Breastfeeding: Risk of Major Malformations, Dose-dependency, Monotherapy vs Polytherapy, Pharmacokinetics and Clinical Implications. Curr Neuropharmacol. 2021;19(11):1805–1824.
24.
Besag F, Vasey MJ. Neurocognitive Effects of Antiseizure Medications in Children and Adolescents with Epilepsy. Paediatric drugs. 2021;23(3):253–286.
25.
Strzelczyk A, Schubert-Bast S. Psychobehavioural and Cognitive Adverse Events of Anti-Seizure Medications for the Treatment of Developmental and Epileptic Encephalopathies. CNS drugs. 2022;36(10):1079–1111.
26.
Hodorovich DR, Lindsley PM, Berry AA, et al. Morphological and sensorimotor phenotypes in a zebrafish CHARGE syndrome model are domain-dependent. Genes Brain Behav. 2023;22(3):e12839.
27.
Oshikoya KA, Carroll R, Aka I, et al. Adverse Events Associated with Risperidone Use in Pediatric Patients: A Retrospective Biobank Study. Drugs Real World Outcomes. 2019;6(2):59–71.
28.
Kanca O, Andrews JC, Lee PT, et al. De Novo Variants in WDR37 Are Associated with Epilepsy, Colobomas, Dysmorphism, Developmental Delay, Intellectual Disability, and Cerebellar Hypoplasia. Am J Hum Genet. 2019;105(2):413–424.
29.
Reis LM, Sorokina EA, Thompson S, et al. De Novo Missense Variants in WDR37 Cause a Severe Multisystemic Syndrome. Am J Hum Genet. 2019;105(2):425–433.
30.
Hay E, Henderson RH, Mansour S, et al. Expanding the phenotypic spectrum consequent upon de novo WDR37 missense variants. Clin Genet. 2020;98(2):191–197.
31.
Samejima M, Nakashima M, Shibasaki J, et al. Splicing variant of WDR37 in a case of Neurooculocardiogenitourinary syndrome. Brain Dev. 2024;46(3):154–159.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.