Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
2025
General Data Protection Regulation (RODO)
REVIEW PAPER
Current state of melanoma treatment – from conventional therapies to nanotechnology and beyond
1
Department of Medical Chemistry, Medical University of Lublin, Lublin, Poland
2
Department of Biochemistry and Molecular Biology, Medical University of Lublin, Lublin, Poland
Corresponding author
Ewa Błaszczak
Department of Biochemistry and Molecular Biology, Medical University of Lublin, W. Chodzki 1, 20-093 Lublin, Poland
Department of Biochemistry and Molecular Biology, Medical University of Lublin, W. Chodzki 1, 20-093 Lublin, Poland
J Pre Clin Clin Res. 2024;18(4):333-340
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Melanoma is recognized as the most aggressive type of skin cancer, and its global incidence is rising. Early detection of melanoma is crucial, as it allows for curative surgical removal with clear margins based on the tumour’s depth. However, managing advanced melanoma, particularly cases with metastasis, remains a significant clinical challenge, often leading to fatal outcomes. The aim of the review is to highlight the current knowledge of melanoma treatment strategies, with a focus on both conventional therapies and recent advancements, including immunotherapy and nanotechnology-based approaches
Review methods:
The literature review made use of databases including PubMed and Google Scholar, with the sources ranging from 2017–2024. Key words included primarily ‘melanoma’, ‘melanoma treatment’ and ‘melanoma therapy’. Peerreviewed articles were included, both reviews and original research papers involving cell lines, animal models, and patient cohorts.
Brief description of the state of knowledge:
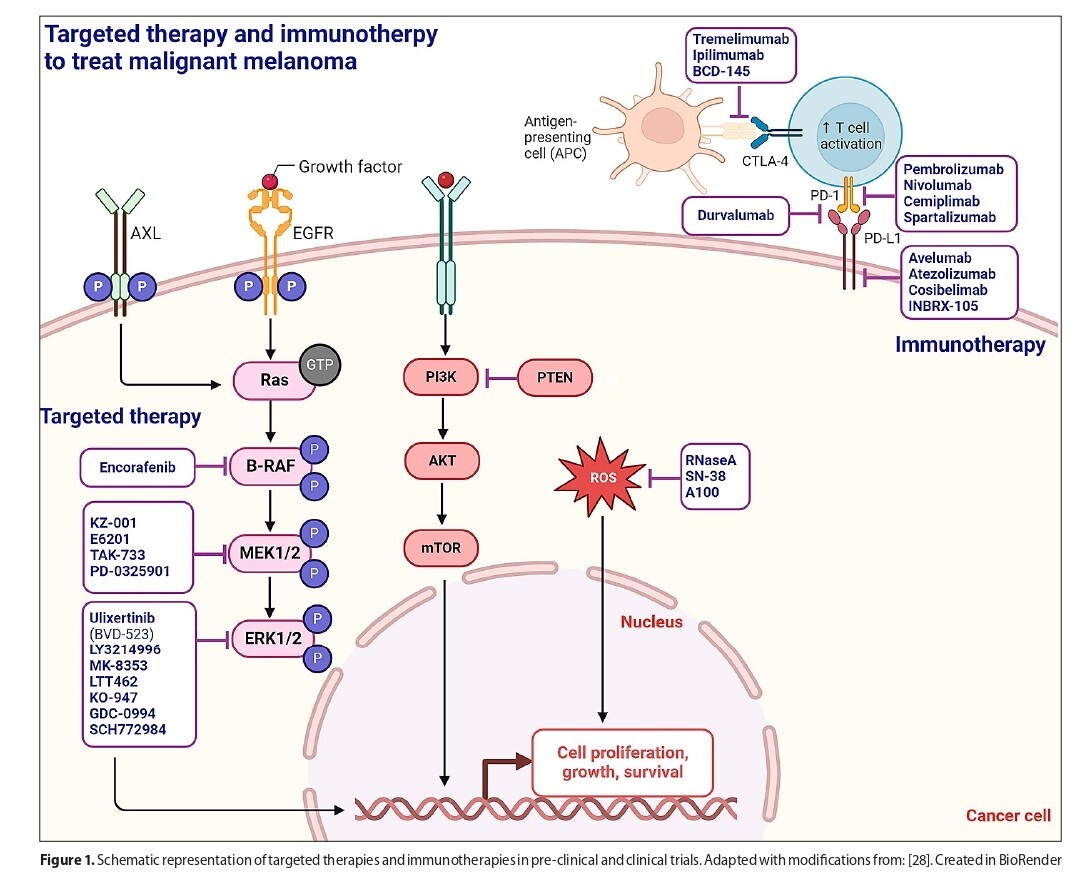
Treatment such as radiotherapy and chemotherapy face such challenges as resistance, leading to melanoma recurrence and progression, along with side-effects. Recent advancements focus on more targeted and personalised treatments. Targeted therapies and immunotherapies, particularly with immune checkpoint inhibitors, have shown considerable potential, although they also come with limitations. Hence, innovative approaches, including the use of nanotechnology and combination therapies, are being developed to further enhance melanoma treatment.
Summary:
The significant metastatic capacity of melanoma, the poor prognosis associated with its advanced stages, and the limitations of conventional therapies, emphasise the need for novel treatment strategies.
Melanoma is recognized as the most aggressive type of skin cancer, and its global incidence is rising. Early detection of melanoma is crucial, as it allows for curative surgical removal with clear margins based on the tumour’s depth. However, managing advanced melanoma, particularly cases with metastasis, remains a significant clinical challenge, often leading to fatal outcomes. The aim of the review is to highlight the current knowledge of melanoma treatment strategies, with a focus on both conventional therapies and recent advancements, including immunotherapy and nanotechnology-based approaches
Review methods:
The literature review made use of databases including PubMed and Google Scholar, with the sources ranging from 2017–2024. Key words included primarily ‘melanoma’, ‘melanoma treatment’ and ‘melanoma therapy’. Peerreviewed articles were included, both reviews and original research papers involving cell lines, animal models, and patient cohorts.
Brief description of the state of knowledge:
Treatment such as radiotherapy and chemotherapy face such challenges as resistance, leading to melanoma recurrence and progression, along with side-effects. Recent advancements focus on more targeted and personalised treatments. Targeted therapies and immunotherapies, particularly with immune checkpoint inhibitors, have shown considerable potential, although they also come with limitations. Hence, innovative approaches, including the use of nanotechnology and combination therapies, are being developed to further enhance melanoma treatment.
Summary:
The significant metastatic capacity of melanoma, the poor prognosis associated with its advanced stages, and the limitations of conventional therapies, emphasise the need for novel treatment strategies.
ACKNOWLEDGEMENTS
This figure was created using BioRender.com (adapted with
modifications from template [28]) under a granted license.
Blaszczak, E. (2024) BioRender.com/e31a878.
Krasowska D, Kurzepa J, Błaszczak E. Current state of melanoma treatment: from conventional therapies to nanotechnology and beyond. J Pre-Clin Clin Res. 2024; 18(4): 333–340. doi: 10.26444/jpccr/193990
REFERENCES (82)
1.
Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet. 2018;392:971–84.
2.
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
3.
Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A. Epidemiology of Melanoma. Med Sci (Basel). 2021;9. https://doi.org/10.3390/ medsci9040063.
4.
Jager MJ, Shields CL, Cebulla CM, Abdel-Rahman MH, Grossniklaus HE, Stern M-H, et al. Uveal melanoma. Nat Rev Dis Primers. 2020;6:24.
5.
Nassar KW, Tan AC. The mutational landscape of mucosal melanoma. Semin Cancer Biol. 2020;61:139–48.
6.
Guo P, Wei X, Guo Z, Wu D. Clinicopathological features, current status, and progress of primary central nervous system melanoma diagnosis and treatment. Pigment Cell Melanoma Res. 2023. https:// doi.org/10.1111/pcmr.13140.
7.
Lotz M, Budden T, Furney SJ, Virós A. Molecular subtype, biological sex and age shape melanoma tumour evolution. Br J Dermatol. 2021;184:328–37.
8.
Casula M, Paliogiannis P, Ayala F, De Giorgi V, Stanganelli I, Mandalà M, et al. Germline and somatic mutations in patients with multiple primary melanomas: a next generation sequencing study. BMC Cancer. 2019;19:772.
9.
Ticha I, Hojny J, Michalkova R, Kodet O, Krkavcova E, Hajkova N, et al. A comprehensive evaluation of pathogenic mutations in primary cutaneous melanomas, including the identification of novel loss-offunction variants. Sci Rep. 2019;9:17050.
10.
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80.
11.
Heppt MV, Siepmann T, Engel J, Schubert-Fritschle G, Eckel R, Mirlach L, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
12.
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–92.
13.
Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18:775–84.
14.
Joyce D, Skitzki JJ. Surgical management of primary cutaneous melanoma. Surg Clin North Am. 2020;100:61–70.
15.
Long GV, Swetter SM, Menzies AM, Gershenwald JE, Scolyer RA. Cutaneous melanoma. Lancet. 2023;402:485–502.
16.
Switzer B, Puzanov I, Skitzki JJ, Hamad L, Ernstoff MS. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol Pract. 2022;18:335–51.
17.
Xu M, Li S. Nano-drug delivery system targeting tumor microenvironment: A prospective strategy for melanoma treatment. Cancer Lett. 2023;574:216397.
18.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–46.
19.
Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–36.
20.
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–8.
21.
Liszewski W, Stewart JR, Vidal NY, Demer AM. Incisional biopsy technique is ass ociated with decreased overall survival for cutaneous melanoma. Dermatol Surg. 2022;48:486–91.
22.
Dixon AJ, Sladden M, Zouboulis CC, Popescu CM, Nirenberg A, Steinman HK, et al. Primary cutaneous melanoma-management in 2024. J Clin Med Res. 2024;13:1607.
23.
Shah C, Punglia R. Sentinel Lymph Node Biopsy in Melanoma: The Role of Tumor Thickness and Histopathologic Features. Surg Oncol Clin N Am. 2017;26:379–89.
24.
Gershenwald JE, Sondak VK, Mcmasters KM. Sentinel Lymph Node Biopsy for Melanoma: A Clinical Practice Guideline. J Clin Oncol. 2020;38:3684–96.
25.
Shi W, Department of Radiation Oncology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA 19107, USA. Radiation Therapy for Melanoma. Cutaneous Melanoma: Etiology and Therapy, Codon Publications; 2017, p. 101–20.
26.
Pham JP, Joshua AM, da Silva IP, Dummer R, Goldinger SM. Chemotherapy in Cutaneous Melanoma: Is There Still a Role? Curr Oncol Rep. 2023;25:609–21.
27.
Jenkins RW, Fisher DE. Treatment of Advanced Melanoma in 2020 and Beyond. J Invest Dermatol 2021;141:23–31.
28.
Patel H, Yacoub N, Mishra R, White A, Long Y, Alanazi S, et al. Current advances in the treatment of BRAF-mutant melanoma. Cancers (Basel). 2020;12:482.
29.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
30.
Larkin J, Brown MP, Arance AM, Hauschild A, Queirolo P, Vecchio MD, et al. An open-label, multicentre safety study of vemurafenib in patients with BRAFV600-mutant metastatic melanoma: final analysis and a validated prognostic scoring system. Eur J Cancer. 2019;107:175–85.
31.
Hauschild A, Ascierto PA, Schadendorf D, Grob JJ, Ribas A, Kiecker F, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib monotherapy: Analysis from phase 2 and 3 clinical trials. Eur J Cancer. 2020;125:114–20.
32.
Okten IN, Ismail S, Withycombe BM, Eroglu Z. Preclinical discovery and clinical development of encorafenib for the treatment of melanoma. Expert Opin Drug Discov. 2020;15:1373–80.
33.
Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315–27.
34.
Tian Y, Guo W. A Review of the Molecular Pathways Involved in Resistance to BRAF Inhibitors in Patients with Advanced-Stage Melanoma. Med Sci Monit. 2020;26:e920957.
35.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16.
36.
Gouda MA, Subbiah V. Expanding the benefit: Dabrafenib/trametinib as tissue-agnostic therapy for BRAF V600E-positive adult and pediatric solid tumors. Am Soc Clin Oncol Educ Book. 2023;43:e404770.
37.
Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76.
38.
Mao L, Dai J, Cao Y, Bai X, Sheng X, Chi Z, et al. Palbociclib in advanced acral melanoma with genetic aberrations in the cyclin-dependent kinase 4 pathway. Eur J Cancer 2021;148:297–306.
39.
Luke JJ, Rutkowski P, Queirolo P, Del Vecchio M, Mackiewicz J, Chiarion-Sileni V, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718–29.
40.
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40:127–37.
41.
Pires da Silva I, Ahmed T, Reijers ILM, Weppler AM, Betof Warner A, Patrinely JR, et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 2021;22:836–47.
42.
Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. JCO. 2015;33:2780–8.
44.
Phadke MS, Smalley KSM. Targeting NRAS mutations in advanced melanoma. J Clin Oncol. 2023;41:2661–4.
45.
Callahan MK, Chapman PB. PD-1 or PD-L1 blockade adds little to combination of BRAF and MEK inhibition in the treatment of BRAF V600-mutated melanoma. J Clin Oncol. 2022;40:1393–5.
46.
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65.
47.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51.
48.
Hodi FS, Chiarion -Sileni V, Lewis KD, Grob J-J, Rutkowski P, Lao CD, et al. Long-term survival in advanced melanoma for patients treated with nivolumab plus ipilimumab in CheckMate 067. J Clin Oncol. 2022;40:9522–9522.
49.
Brahmer JR, Lacchetti C, Schneider BJ. For personal use only. No other uses without permission. Copyright © 2021 Massachusetts Medical Society. All rights reserved. 2240 n engl j med 384. J Clin Oncol. 2018;23:1714–68.
50.
Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–30.
51.
Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201.
52.
Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer. 2019;19:587–602.
53.
Gao D, Guo X, Zhang X, Chen S, Wang Y, Chen T, et al. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater Today Bio. 2020;5:100035.
54.
Yadav N, Dahiya T, Chhillar AK, Rana JS, Saini HM. Nanotechnology in cancer diagnostics and therapeutics: A review. Curr Pharm Biotechnol. 2022;23:1556–68.
55.
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol 2019;71:1185–98.
56.
Bensa V, Calarco E, Giusto E, Perri P, Corrias MV, Ponzoni M, et al. Retinoids delivery systems in cancer: Liposomal fenretinide for neuroectodermal-derived tumors. Pharmaceuticals. 2021;14:854.
57.
Gmeiner WH, Ghosh S. Nanotechnology for cancer treatment. Nanotechnol Rev. 2015;3:111–22.
58.
Kurakula M, Chen L, Tiwari AK, Srinivas NR, Dash RP, Panizzi PR, et al. Recent advances in lipid-based nanovesicular delivery systems for melanoma therapy. Crit Rev Ther Drug Carrier Syst. 2021;38:1–38.
59.
Ghosh B, Biswas S. Polymeric micelles in cancer therapy: State of the art. J Control Release. 2021;332:127–47.
60.
Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, et al. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv 2020;10:26777–91.
61.
Xiong W, Guo Z, Zeng B, Wang T, Zeng X, Cao W, et al. Dacarbazine-Loaded Targeted Polymeric Nanoparticles for Enhancing Malignant Melanoma Therapy. Front Bioeng Biotechnol. 2022;10. https://doi.org/10.3389/fbioe.....
62.
Chowdhury A, Kunjiappan S, Panneerselvam T, Somasundaram B, Bhattacharjee C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int Nano Lett. 2017;7:91–122.
63.
Zhang Y, Zhan X, Xiong J, Peng S, Huang W, Joshi R, et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci Rep. 2018;8. https://doi.org/10.1038/s41598....
64.
Zielińska A, Pereira I, Antunes S, Veiga FJ, Santos AC, Nowak I, et al. Mesoporous silica nanoparticles as drug delivery systems against melanoma. In: Grumezescu AM, editor. Design of Nanostructures for Theranostics Applications. Elsevier; 2018. p. 437–66.
65.
Karami Fath M, Azargoonjahromi A, Jafari N, Mehdi M, Alavi F, Daraei M, et al. Exosome application in tumorigenesis: diagnosis and treatment of melanoma. Med Oncol. 2022;39:19.
66.
Thuy LT, Kang N, Choi M, Lee M, Choi JS. Dendrimeric micelles composed of polyamidoamine dendrimer-peptide-cholesterol conjugates as drug carriers for the treatment of melanoma and bacterial infection. J Ind Eng Chem. 2022;114:361–76.
67.
Huang P-C, Chaney EJ, Aksamitiene E, Barkalifa R, Spillman DR Jr, Bogan BJ, et al. Biomechanical sensing of in vivo magnetic nanoparticle hyperthermia-treated melanoma using magnetomotive optical coherence elastography. Theranostics. 2021;11:5620–33.
68.
Bedikian AY, Vardeleon A, Smith T, Campbell S, Namdari R. Pharmacokinetics and urinary excretion of vincristine sulfate liposomes injection in metastatic melanoma patients. J Clin Pharmacol. 2006;46:727–37.
69.
Gargett T, Abbas MN, Rolan P, Price JD, Gosling KM, Ferrante A, et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol Immunother. 2018;67:1461–72.
70.
Zeng H, Li J, Hou K, Wu Y, Chen H, Ning Z. Melanoma and nanotechnology-based treatment. Front Oncol. 2022;12:858185.
71.
Ni D, Jiang D, Ehlerding EB, Huang P, Cai W. Radiolabeling silica-based nanoparticles via coordination chemistry: Basic principles, strategies, and applications. Acc Chem Res. 2018;51:778–88.
72.
Zou Y, Wei Y, Sun Y, Bao J, Yao F, Li Z, et al. Cyclic RGD-functionalized and disulfide-crosslinked iodine-rich polymersomes as a robust and smart theranostic agent for targeted CT imaging and chemotherapy of tumor. Theranostics. 2019;9:8061–72.
73.
Wang M, Geilich BM, Keidar M, Webster TJ. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int J Nanomedicine. 2017;12:4117–27.
74.
Turan IS, Yildiz D, Turksoy A, Gunaydin G, Akkaya EU. A bifunctional photosensitizer for enhanced fractional photodynamic therapy: Singlet oxygen generation in the presence and absence of light. Angew Chem Int Ed Engl. 2016;55:2875–8.
75.
Lara-Vega I, Vega-López A. Combinational photodynamic and photothermal – based therapies for melanoma in mouse models. Photodiagnosis Photodyn Ther. 2023;43:103596.
76.
Zhao L, Zhang X, Wang X, Guan X, Zhang W, Ma J. Recent advances in selective photothermal therapy of tumor. J Nanobiotechnology. 2021;19:335.
77.
Baldea I, Giurgiu L, Teacoe ID, Olteanu DE, Olteanu FC, Clichici S, et al. Photodynamic Therapy in Melanoma – Where do we Stand? Curr Med Chem. 2018;25:5540–63.
78.
Dheyab MA, Aziz AA, Khaniabadi PM, Jameel MS. Potential of a sonochemical approach to generate MRI-PPT theranostic agents for breast cancer. Photodiagnosis Photodyn Ther. 2021;33:102177.
79.
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98:8554–9.
80.
Schneekloth JS Jr, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, et al. Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc. 2004;126:3748–54.
81.
Ishida T, Ciulli A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS Discovery. 2021;26:484–502.
82.
Rathod D, Fu Y, Patel K. BRD4 PROTAC as a novel therapeutic approach for the treatment of vemurafenib resistant melanoma: Preformulation studies, formulation development and in vitro evaluation. Eur J Pharm Sci. 2019;138:105039.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.