REVIEW PAPER
Could glutamate be a diagnostic marker in neurological and psychiatric diseases?
1
Student Research Group ISOMERS at the Department of Medicinal Chemistry, Medical University, Lublin, Poland
2
Department of Medical Chemistry, Medical University, Lublin, Poland
Corresponding author
Dorota Luchowska-Kocot
Department of Medical Chemistry, Medical University of Lublin, 4A Chodźki Street, 20-093, Lublin, Poland
Department of Medical Chemistry, Medical University of Lublin, 4A Chodźki Street, 20-093, Lublin, Poland
J Pre Clin Clin Res. 2025;19(1):20-28
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
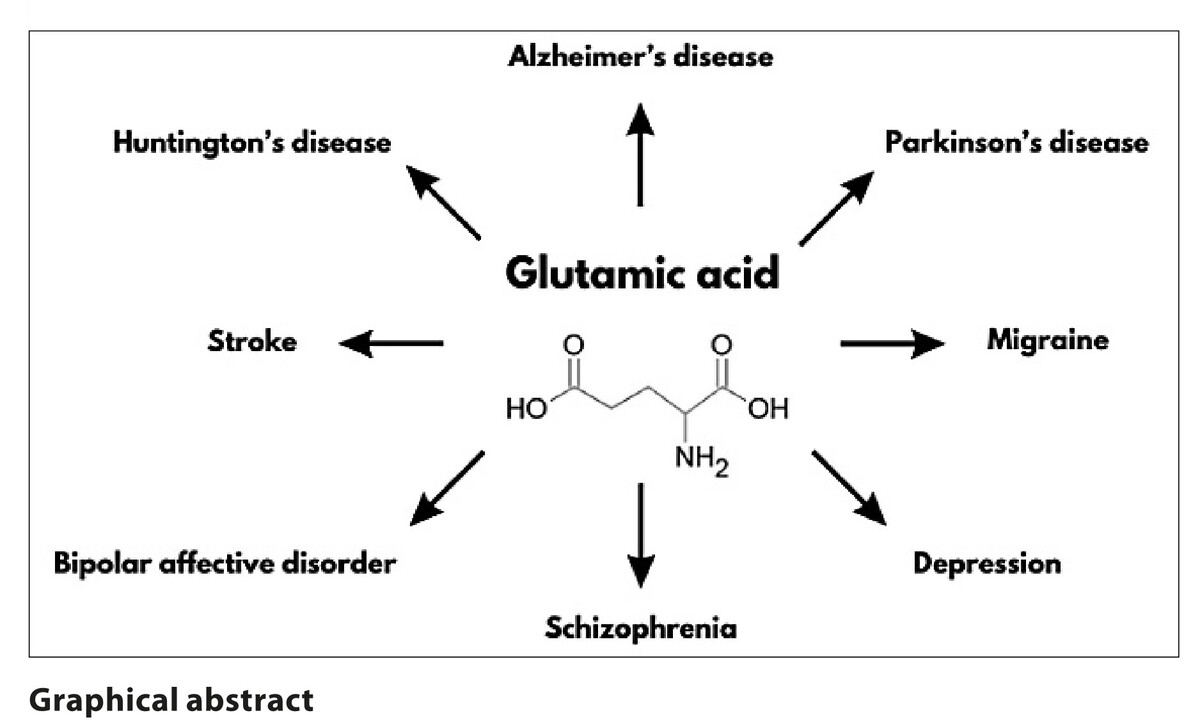
Glutamate plays a role in the pathogenesis of numerous neurological disorders, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, migraine, and stroke. Additionally, it is implicated in the aetiology of psychiatric disorders, such as schizophrenia, depression, and bipolar affective disorder. As it can be simply identified in bodily fluids, fluctuations in its levels may serve as a potential indicator of pathological processes. The aim of the study was to determine whether fluctuations in glutamate concentrations could be beneficial in predicting and monitoring the progression of the mentioned diseases.
Review methods:
A literature search was conducted in the PubMed database using the following algorithm: (glutamate) AND (blood/plasma/serum/nerve tissue) AND (neurodegeneration/Alzheimer/Parkinson/migraine/stroke/psychiatric/depression/schizophrenia). More than 80% of the identified publications were published in 2017 or later.
Brief description of the state of knowledge:
The majority of studies cited revealed a clear difference in glutamate concentrations between the control group and the study group. In the majority of cases of neurodegenerative diseases, blood glutamate concentrations demonstrated a downward trend. Conversely, in psychiatric diseases, stroke and migraine, they exhibited an upward trend.
Summary:
Damage to the blood-brain barrier, which regulates glutamate transfer from nerve tissue to blood, appears to significantly affect glutamate levels in both blood and nerve tissue during disease. Altered blood glutamate concentration could serve as a diagnostic marker, although meta-analysis is needed to define clinically applicable ranges.
Glutamate plays a role in the pathogenesis of numerous neurological disorders, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, migraine, and stroke. Additionally, it is implicated in the aetiology of psychiatric disorders, such as schizophrenia, depression, and bipolar affective disorder. As it can be simply identified in bodily fluids, fluctuations in its levels may serve as a potential indicator of pathological processes. The aim of the study was to determine whether fluctuations in glutamate concentrations could be beneficial in predicting and monitoring the progression of the mentioned diseases.
Review methods:
A literature search was conducted in the PubMed database using the following algorithm: (glutamate) AND (blood/plasma/serum/nerve tissue) AND (neurodegeneration/Alzheimer/Parkinson/migraine/stroke/psychiatric/depression/schizophrenia). More than 80% of the identified publications were published in 2017 or later.
Brief description of the state of knowledge:
The majority of studies cited revealed a clear difference in glutamate concentrations between the control group and the study group. In the majority of cases of neurodegenerative diseases, blood glutamate concentrations demonstrated a downward trend. Conversely, in psychiatric diseases, stroke and migraine, they exhibited an upward trend.
Summary:
Damage to the blood-brain barrier, which regulates glutamate transfer from nerve tissue to blood, appears to significantly affect glutamate levels in both blood and nerve tissue during disease. Altered blood glutamate concentration could serve as a diagnostic marker, although meta-analysis is needed to define clinically applicable ranges.
Kwiatkowski B, Biedroń N, Gawryś U, Tochman W, Luchowska-Kocot D. Could glutamate be a diagnostic marker in neurological and psychiatric
diseases? J Pre-Clin Clin Res. 2025; 19(1): 20–28. doi: 10.26444/jpccr/201214
REFERENCES (68)
1.
Cynober L. Metabolism of Dietary Glutamate in Adults. Ann Nutr Metab. 2018;73 (Suppl 5):5–14.
2.
Brosnan JT, Brosnan ME. Glutamate: a truly functional amino acid. Amino Acids. 2013;45(3):413–418.
3.
Sánchez-Cano F, Hernández-Kelly LC, Ortega A. Silica Nanoparticles Decrease Glutamate Uptake in Blood-Brain Barrier Components. Neurotox Res. 2024;42(2):20.
4.
Neves D, Salazar IL, Almeida RD, et al. Molecular mechanisms of ischemia and glutamate excitotoxicity. Life Sci. 2023;328:121814.
5.
Sears SMS, Hewett SJ. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp Biol Med (Maywood). 2021 May;246(9):1069–1083.
6.
Liu X, Tong X, Zou Y, et al. Mendelian randomization analyses support causal relationships between blood metabolites and the gut microbiome. Nat Genet. 2022;54(1):52–61.
7.
Jiang W, Gong L, Liu F, et al. Alteration of Gut Microbiome and Correlated Lipid Metabolism in Post-Stroke Depression. Front Cell Infect Microbiol. 2021;11:663967.
8.
Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17(1):69.
9.
Hladky SB, Barrand MA. Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood–brain barrier. Fluids and Barriers of the CNS. 2018;15(1):30.
10.
Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19.
11.
Gruenbaum BF, Zlotnik A, Fleidervish I, et al. Glutamate Neurotoxicity and Destruction of the Blood–Brain Barrier: Key Pathways for the Development of Neuropsychiatric Consequences of TBI and Their Potential Treatment Strategies. Int J Mol Sci. 2022;23(17):9628.
12.
Schultz J, Uddin Z, Singh G, et al. Correction: Glutamate sensing in biofluids: recent advances and research challenges of electrochemical sensors. Analyst. 2020;145(12):4369–4371.
13.
Ino H, Honda S, Yamada K, et al. Glutamatergic Neurometabolite Levels in Bipolar Disorder: A Systematic Review and Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8(2):140–150.
14.
Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). 2018;243(3):213–221.
15.
Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1041.
16.
Czapski GA, Strosznajder JB. Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer’s Disease. Int J Mol Sci. 2021;22(21):11677.
17.
Andersen JV, Markussen KH, Jakobsen E, et al. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology. 2021;196:108719.
18.
Wang H, Tan L, Wang HF, et al. Magnetic Resonance Spectroscopy in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J Alzheimers Dis. 2015 Jun 26;46(4):1049–1070.
19.
Smucny J, Maddock RJ. Spectroscopic meta-analyses reveal novel metabolite profiles across methamphetamine and cocaine substance use disorder. Drug Alcohol Depend. 2023;248:109900.
20.
Morland C, Nordengen K. N-Acetyl-Aspartyl-Glutamate in Brain Health and Disease. Int J Mol Sci. 2022;23(3):1268.
21.
Wong D, Atiya S, Fogarty J, et al. Reduced Hippocampal Glutamate and Posterior Cingulate N-Acetyl Aspartate in Mild Cognitive Impairment and Alzheimer’s Disease Is Associated with Episodic Memory Performance and White Matter Integrity in the Cingulum: A Pilot Study. J Alzheimers Dis. 2020;73(4):1385–1405.
22.
Zeydan B, Deelchand DK, Tosakulwong N, et al. Decreased glutamate levels in patients with amnestic mild cognitive impairment: an sLASER proton MR spectroscopy and PiB-PET study. J Neuroimaging. 2017;27(6):630–636.
23.
Kara F, Kantarci K. Understanding Proton Magnetic Resonance Spectroscopy Neurochemical Changes Using Alzheimer’s Disease Biofluid, PET, Postmortem Pathology Biomarkers, and APOE Genotype. Int J Mol Sci. 2024;25(18):10064.
24.
Buard I, Lopez-Esquibel N, Carey FJ, et al. Does Prefrontal Glutamate Index Cognitive Changes in Parkinson’s Disease? Front Hum Neurosci. 2022;16:809905.
25.
Fiandaca MS, Gross TJ, Johnson TM, et al. Potential Metabolomic Linkage in Blood between Parkinson’s Disease and Traumatic Brain Injury. Metabolites. 2018;8(3):50.
26.
Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, et al. Cerebrospinal and blood levels of amino acids as potential biomarkers for Parkinson’s disease: review and meta-analysis. Eur J Neurol. 2020;27(11):2336–2347.
27.
Pépin J, Francelle L, Carrillo-de Sauvage MA, et al. In vivo imaging of brain glutamate defects in a knock-in mouse model of Huntington’s disease. Neuroimage. 2016;139:53–64.
28.
Vijayakumar UG, Milla V, Stafford MYC, et al. A systematic review of suggested molecular strata, biomarkers and their tissue sources in ALS. Front Neurol. 2019;10(MAY):400.
29.
Peters GL. Migraine overview and summary of current and emerging treatment options. Am J Manag Care. 2019;25(2 Suppl):S23–34.
30.
Gao X, Wang J. Quantitative assessment of the association between GRIA1 polymorphisms and migraine risk. Biosci Rep. 2018;38(6):BSR20181347.
31.
Tripathi GM, Kalita J, Misra UK. Role of glutamate and its receptors in migraine with reference to amitriptyline and transcranial magnetic stimulation therapy. Brain Res. 2018;1696:31–37.
32.
Cho S, Chu MK. Serological Biomarkers of Chronic Migraine. Curr Pain Headache Rep. 2023;27(10):531–542.
33.
Park CG, Chu MK. Interictal plasma glutamate levels are elevated in individuals with episodic and chronic migraine. Sci Rep. 2022;12(1):6921.
34.
Ferrari A, Spaccapelo L, Pinetti D, et al. Effective prophylactic treatments of migraine lower plasma glutamate levels. Cephalalgia. 2009 Apr;29(4):423–429.
35.
da Silva-Candal A, Pérez-Díaz A, Santamaría M, et al. Clinical validation of blood/brain glutamate grabbing in acute ischemic stroke. Ann Neurol. 2018;84(2):260–273.
36.
Wen L, Yan C, Zheng W, et al. Metabolic Alterations and Related Biological Functions of Post-Stroke Depression in Ischemic Stroke Patients. Neuropsychiatr Dis Treat. 2023;19:1555–1564.
37.
Cheng SY, Zhao YD, Li J, et al. Plasma levels of glutamate during stroke is associated with development of post-stroke depression. Psychoneuroendocrinology. 2014;47:126–135.
38.
Li T, Luo HH, Feng XF, et al. Plasma Free Amino Acids and Risk of Cardiovascular Disease in Chinese Patients With Type 2 Diabetes. Front Endocrinol (Lausanne). 2021;11:519923.
39.
Zhu Z, Yang P, Jia Y, et al. Plasma Amino Acid Neurotransmitters and Ischemic Stroke Prognosis: A Multicenter Prospective Study. Am J Clin Nutr. 2023;118(4):754–762.
40.
Coyle JT. Passing the torch: The ascendance of the glutamatergic synapse in the pathophysiology of schizophrenia. Biochem Pharmacol. 2024;228:116376.
41.
Okubo R, Okada M, Motomura E. Dysfunction of the NMDA Receptor in the Pathophysiology of Schizophrenia and/or the Pathomechanisms of Treatment-Resistant Schizophrenia. Biomolecules. 2024;14(9):1128.
42.
Haaf M, Curic S, Rauh J, et al. Opposite Modulation of the NMDA Receptor by Glycine and S-Ketamine and the Effects on Resting State EEG Gamma Activity: New Insights into the Glutamate Hypothesis of Schizophrenia. Int J Mol Sci. 2023;24(3):1913.
43.
Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427.
44.
Kruse AO, Bustillo JR. Glutamatergic dysfunction in Schizophrenia. Transl Psychiatry. 2022;12(1):500.
45.
Madeira C, Alheira F V., Calcia MA, et al. Blood Levels of Glutamate and Glutamine in Recent Onset and Chronic Schizophrenia. Front Psychiatry. 2018;9:713.
46.
Ivanova SA, Boyko AS, Fedorenko OYu, et al. Glutamate Concentration in the Serum of Patients with Schizophrenia. Procedia Chem. 2014;10:80–85.
47.
Song J, Viggiano A, Monda M, et al. Peripheral glutamate levels in schizophrenia: evidence from a meta-analysis. Neuropsychobiology. 2014;70(3):133–141.
48.
Martino M, Magioncalda P. Tracing the psychopathology of bipolar disorder to the functional architecture of intrinsic brain activity and its neurotransmitter modulation: a three-dimensional model. Mol Psychiatry. 2022;27(2):793–802.
49.
Guglielmo R, Hasler G. The neuroprotective and neuroplastic potential of glutamatergic therapeutic drugs in bipolar disorder. Neurosci Biobehav Rev. 2022;142:104906.
50.
Pålsson E, Jakobsson J, Södersten K, et al. Markers of glutamate signaling in cerebrospinal fluid and serum from patients with bipolar disorder and healthy controls. Eur Neuropsychopharmacol. 2015;25(1):133–140.
51.
Liao JW, Wang SS, Yang HH, et al. [Comparative analysis of serum glutamate and gamma-aminobutyric acid levels in patients with bipolar depressive disorder and major depression disorder]. Zhonghua Yi Xue Za Zhi. 2020;100(23):1800–1804.
52.
Inoshita M, Umehara H, Watanabe SY, et al. Elevated peripheral blood glutamate levels in major depressive disorder. Neuropsychiatr Dis Treat. 2018;14:945–953.
53.
Mlyniec K. Zinc in the Glutamatergic Theory of Depression. Curr Neuropharmacol. 2015;13(4):505–513.
54.
Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron. 2019;102(1):75–90.
55.
Guo J, Wang J, Sun W, et al. The advances of post-stroke depression: 2021 update. J Neurol. 2022;269(3):1236–1249.
56.
Chang CH, Lin CH, Liu CY, et al. Plasma d-glutamate levels for detecting mild cognitive impairment and Alzheimer’s disease: Machine learning approaches. J Psychopharmacol. 2021;35(3):265–272.
57.
Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr. 2009;90(3):867S–874S.
58.
Gaggini M, Carli F, Rosso C, et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67(1):145–158.
59.
Nagao H, Nishizawa H, Fukuda S, et al. Correlation between plasma glutamate and adiponectin in patients with type 2 diabetes. Endocr J. 2024;71(1):55–63.
60.
Onaolapo AY, Onaolapo OJ. Dietary glutamate and the brain: In the footprints of a Jekyll and Hyde molecule. Neurotoxicology. 2020;80:93–104.
61.
Dong R, Denier-Fields DN, Van Hulle CA, et al. Identification of plasma metabolites associated with modifiable risk factors and endophenotypes reflecting Alzheimer’s disease pathology. European Journal of Epidemiology. 2023;38(5):559–571.
62.
Lin CH, Yang HT, Chiu CC, et al. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci Rep. 2017;7(1):14849.
63.
Andreadou E, Kapaki E, Kokotis P, et al. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis: The effect of riluzole treatment. Clin Neurol Neurosurg. 2008;110(3):222–226.
64.
Van Dongen RM, Zielman R, Noga M, et al. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia. 2017;37(1):49–63.
65.
Campos F, Sobrino T, Pérez-Mato M, et al. Glutamate oxaloacetate transaminase: a new key in the dysregulation of glutamate in migraine patients. Cephalalgia. 2013;33(14):1148–1154.
66.
Merritt K, McCutcheon RA, Aleman A, et al. Variability and magnitude of brain glutamate levels in schizophrenia: a meta and mega-analysis. Mol Psychiatry. 2023;28(5):2039–2048.
67.
Galińska-Skok B, Waszkiewicz N. Markers of Schizophrenia—A Critical Narrative Update. J Clin Med. 2022;11(14):3964.
68.
Shen J, Tomar JS. Elevated Brain Glutamate Levels in Bipolar Disorder and Pyruvate Carboxylase-Mediated Anaplerosis. Front Psychiatry. 2021;12:640977.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.